History of Hot Galvanizing
History of Hot-Dip Galvanizing
1)Initial discovery and scientific foundations
- 18th Century: The first experiments in coating metals with zinc were conducted, particularly by the French chemist Paul Jacques Malouin in 1742. He observed that by dipping iron into molten zinc, a protective layer could be created on it.
- 1836: Stanislas Sorel, a French scientist, scientifically improved this process and in 1837 registered the first official patent for hot-dip galvanizing. He used the term “galvanizing” (derived from the name of the Italian scientist Luigi Galvani) to describe this method.
2) Industrial expansion in the 19th and 20th centuries
- After the Industrial Revolution, the demand for corrosion-resistant steel (especially in railways, bridges, and shipbuilding) increased.
- Late 19th century: Galvanizing factories were established in Europe and Amerika, and this method was widely used in construction projects.
- World War I and II: The demand for galvanized steel in the construction of military equipment, tanks, and marine structures surged significantly.
3)Technological advances in recent decades
- Today, the hot-dip galvanizing process has become a standard method with precise control over temperature, immersion time, and the chemical composition of the molten zinc.
- The use of advanced zinc-iron alloys (such as ZAM or Galfan) has increased the corrosion resistance and flexibility of the coating.
- Modern applications: This method is used in the construction of power transmission towers, road guardrails, wind turbines, oil and gas industries, and even modern architecture.
4)Key advantages of Hot-Dip Galvanizing
- Long-lasting protection: Lifespan of 50 to 100 years under normal environmental conditions.
- Resistance to atmospheric and saltwater corrosion.
- Cost-effective: More economical compared to other methods such as industrial painting.
- Uniform coating: Even coverage in corners and complex areas.
This technology, after more than 180 years, remains one of the most effective methods for protecting steel in the world
Introduction to Hot-Dip Galvanizing
The hot-dip galvanizing process is an electrochemical method for protecting steel against corrosion by forming zinc-iron alloy layers (Zn-Fe). This method has several scientific and engineering advantages, which are detailed below:
1) Cathodic protection
- Mechanism: Zinc (Zn) has a more negative electrode potential compared to iron (Fe) (according to the galvanic series). When galvanized steel is exposed to a corrosive environment, zinc acts as a sacrificial anode and prevents iron from corroding by oxidizing itself.
Zn→Zn+2e−
- Outcome: Even if the zinc coating gets scratched, the underlying steel will not corrode as long as there is remaining zinc.
2)Formation of resistant alloy layers (Zn-Fe alloy layers)
In the hot-dip galvanizing process, four metallurgical layers are formed, each with different mechanical and corrosion-resistant properties:

These layers gradually transition from steel to pure zinc, providing excellent adhesion of the coating to the steel surface.
3)Resistance to atmospheric and chemical corrosion
- Resistance in various environments:
- Rural environments: Lifespan of 70-100 years
- Industrial/Urban environments: Lifespan of 30-50 years
- Marine environments: Lifespan of 20-40 years
Scientific reason: The alloy layers and the formation of the protective layer (zinc hydroxychloride) on the surface prevent the penetration of corrosive agents.
4) Uniform and complete coating
- In this method, all surfaces (including corners, welds, and complex areas) are coated uniformly.
- Coating thickness: Typically between 50 to 150 microns (depending on immersion time and steel composition).
5) Mechanical durability and wear resistance
- The δ and Γ layers (hard Fe-Zn alloys) have high resistance to impact and wear.
- Comparison with paint: Galvanized coatings do not require frequent maintenance like paint does.
6) Economic efficiency (life-cycle cost)
- The initial cost may be higher than paint, but due to the lack of maintenance and long lifespan, it is more cost-effective in the long run.
- NACE studies: The cost of galvanizing over 30 years is approximately 3 to 5 times lower than that of industrial paint systems.
7)Environmental compatibility
- The zinc used in this process is 100% recyclable.
- Unlike some organic coatings (such as those containing VOCs), hot-dip galvanizing does not produce hazardous pollutants.
Scientific summary of advantages:
- Self-healing cathodic protection
- Multi-layer structure with optimal Fe-Zn composition
- Exceptional resistance to corrosion and wear
- Complete coating without weak points
- Long lifespan and reduced life cycle costs (LCC)
- Environmental compatibility
These features have made hot-dip galvanizing one of the most effective methods for protecting steel in various industries.
Stages of Hot-Dip Galvanizing
The term “hot-dip galvanizing” refers to the process of immersing iron and steel in a bath of molten zinc to create a zinc coating, aimed at increasing the lifespan of iron or steel against corrosion. This coating is formed through a metallurgical process between the molten zinc and the iron present in the steel. It results in a uniform layer of consistent thickness across the entire surface of the metal. This series of operations is illustrated in the figure below.
This technology has been in use for decades and is recognized as a reliable and economical method for protecting metal against corrosion. The three main stages in the hot-dip galvanizing process are:
- Surface preparation
- Galvanization
- Post-galvanization operations
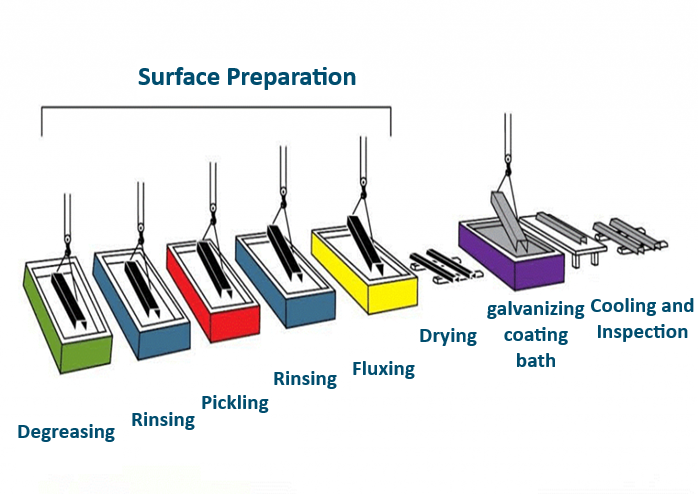
Surface Preparation
Based on the detailed explanation provided, it refers to the initial step in the hot-dip galvanizing process, which involves thoroughly cleaning and preparing the steel surface before the actual galvanizing takes place. The key steps in this surface preparation stage are:
- Degreasing:
- The steel parts are soaked in a bath containing degreasing agents or solvents to remove any oil, grease or other contaminants from the surface.
- After degreasing, the steel is rinsed with water.
- Pickling:
- The steel parts are immersed in a bath containing hydrochloric acid or sulfuric acid.
- This removes any oxidized layers and small stains from the steel surface.
- After pickling, the steel is rinsed with water again.
- Fluxing:
- The purpose of fluxing is to clean any remaining oxides that formed during pickling and create a protective layer on the steel surface.
- This prevents the steel from re-oxidizing before it enters the galvanizing bath.
- The flux solution is slightly acidic and contains a mixture of zinc chloride and ammonium chloride.
After these three crucial surface preparation steps, the steel surface is completely clean and ready for the actual hot-dip galvanizing process in the zinc bath.
Dipping Operation
After the steel surface is fully cleaned and prepared, it is ready to be immersed in the galvanizing bath. The galvanizing bath contains molten zinc that conforms to the ASTM B6 standard, which specifies three different grades of zinc.
Sometimes, other metals may be added to the molten zinc in the galvanizing bath to achieve the desired properties of the galvanized coating. The temperature of the molten zinc in the galvanizing bath is typically between 438-460°C, at which temperature the zinc is in a liquid state.
Once the cleaned steel parts are submerged in the galvanizing bath, they remain there until their temperature reaches a level where the zinc coating can properly form on the steel surface. When the reactions between the zinc and iron are complete, the galvanized steel is removed from the bath.
The entire coating process usually takes less than 10 minutes, with the exact time depending on the thickness of the steel being galvanized.
Post-Galvanizing Operations
After the steel is removed from the galvanizing bath, additional finishing operations may be required to improve the quality of the galvanized coating:
- Rapid cooling:
- The galvanized parts are submerged in a water tank that contains certain chemical additives.
- These additives form a protective layer on the galvanized coating to safeguard the product during transportation and storage.
- Other finishing steps:
- These include removing any excess zinc droplets or spatter on the surface.
- This is typically done by sanding or grinding the parts.
These post-galvanizing operations help enhance the overall quality and appearance of the final galvanized steel product.
Brief introduction of Shimi Faravar Merik Company
Shimi Faravar Merik Company is among the specialized and reputable Iranian companies active in the field of Hot-Dip Galvanizing (HDG) through the immersion process. By utilizing cutting-edge technologies, international standards, and a team of experts, the company has become a trusted supplier for various industries.
Features and advantages of Shimi Faravar Merik Company
- Expertise in high-quality hot-dip galvanizing
• Use of high-purity zinc (compliant with ASTM/ISO standards) for uniform, corrosion-resistant coatings.
• Precise control of process parameters (temperature, immersion time, surface preparation) to ensure optimal results. - Compliance with international standards
• Production aligned with ASTM A123, ASTM A153, ISO 1461, and other industry standards.
• Rigorous quality control tests (e.g., coating thickness, adhesion strength, corrosion resistance). - Advanced equipment & high production capacity
• State-of-the-art hot-dip galvanizing lines capable of coating components of various sizes.
• Expertise in galvanizing heavy structures, pipes, fittings, and industrial/construction components. - Diverse services & customization
• Galvanizing services for industries including:- Oil, gas & petrochemical
- Metal structures & construction
- Power transmission lines & telecommunication towers
- Automotive & railway industries
• Custom galvanizing for components of varying thicknesses per client requirements.
- Commitment to quality & customer satisfaction
• Post-sale technical support and warranty offerings.
• Expert advisory services for optimal metal protection solutions.
Why choose Shimi Faravar Merik?
- High quality and compliance with global standards
- Experienced technical team and reliable after-sales service
- Competitive and cost-effective pricing
- Timely delivery and flexible production
Merik Shimi Faravar is a renowned player in the hot-dip galvanizing industry, known as a trusted partner for both small and large-scale industrial projects.
For more information and to place orders, you can contact the company’s experts:
- Phone:+98 21 8870 8830 , +98 21 8870 6997
• Website: www.merik.ir
• Address: No. 20-21, Arman Street, Lian Street, Persian Gulf Boulevard, Salafchegan Industrial Estate, Qom, Iran
Merik’s technical expertise and commitment to quality make it an ideal choice for industries that require durable and corrosion-resistant solutions.
Hot dip galvanizing services
Galvanized pipes and profiles in various sizes and thicknesses

Galvanized Mannesmann pipes in different sizes and grades

Galvanized electrical conduit pipes

Galvanized profiles for greenhouse industries

Galvanized transmission towers, telecommunication towers, and telescopic structures

Hot-dip galvanized metal equipment and structures used in refinery industries, petrochemical industries and power generation plants

Galvanized high-voltage substation equipment, lighting poles, and road structures (guardrails)

Galvanized steel gratings, cable trays, cable ladders, and other steel structures
